Soothing Stimulation
Deep brain stimulation treats neurological disorders
As 73-year-old Alfredo Hildago was awakened by his neurologist in an operating room at Jackson Memorial Hospital, the tremors from Parkinson’s disease returned to both his hands. But when Bruno V. Gallo, M.D., assistant professor of neurology, activated the electrode just implanted in his brain, the tremors on the right side of his body abruptly stopped.
“Look at this,” Gallo said as he demonstrated the complete flexibility in Hildago’s right arm. “Not only have the tremors in his hand stopped, but the rigidity previously present in
his entire arm is gone as well, and that is even more impressive.”
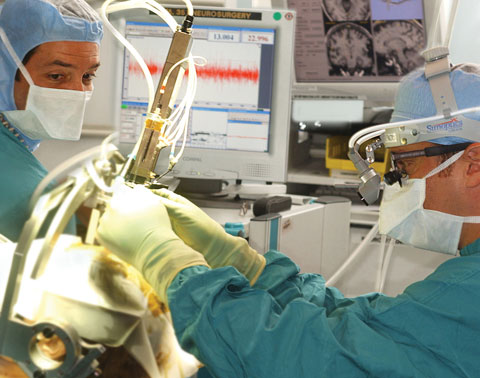 |
| Bruno V. Gallo, M.D., and Jonathan Jagid, M.D., team up on performing deep brain stimulation surgery to treat serious neurological disorders. |
Hildago had just undergone phase one of deep brain stimulation, a surgical procedure used to treat a variety of disabling neurological symptoms, most commonly the symptoms of Parkinson’s, such as tremor, rigidity, stiffness, slowed movement, and walking problems.
“The use of deep brain stimulation is expanding; we now also use it to treat essential tremor and dystonia, and it’s even being tested in clinical trials for depression, epilepsy, and obsessive compulsive disorder,” explains Gallo, who has been involved in more than 500 such procedures during his career.
At the University of Miami/Jackson, Gallo teams with Jonathan Jagid, M.D., assistant professor of neurological surgery. Together, the two perform the procedure twice a week at Jackson Memorial Hospital, and they have completed more than 210 cases. The treatment is a two-step process.
“First, using magnetic resonance imaging we identify the exact target within the brain where electrical nerve signals generate the troubling symptoms, and then we use a metal framing system around the patient’s head
to mark the exact spots where we
need to enter,” says Jagid. “In Hildago’s case, that section of the brain is
the subthalamic nucleus, which is
a pretty common area for patients
with Parkinson’s.”
After Jagid opens a small hole in
the skull and exposes the brain, under only conscious sedation, a microelectrode—a thin wire that acts as a recording device—is passed through
the brain en route to the targeted area
via computer guidance by Gallo, who monitors the electrical activity of the nerve cells on a computer screen to determine when the target area has been reached. The microelectrode is then withdrawn, and Jagid places the permanent stimulating electrode in place.
“In Parkinson’s disease the nerve cells are firing too fast because there
is not enough dopamine to quiet them down,” says Gallo. “We find the area where the cells are on overload and place the electrode to cover that area.”
Once the electrode is in place, the patient is woken up, and the stimulator is activated.
“That is beautiful,” exclaims Gallo as he watches the tremors stop. “You never get used to seeing that happen.”
A week later Hildago will be given general anesthesia so Jagid can pass the electrode under the skin of his head, neck, and shoulder and connect it
to the neurostimulator or the “battery pack” inserted under his collarbone, much like a cardiac pacemaker.
Hildago was the first patient in South Florida to receive a new neurostimulator, which is about 20 percent smaller than previous models.
|