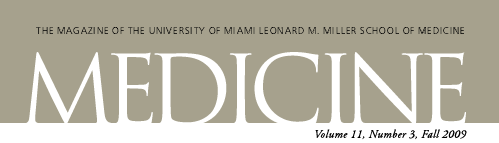|
 |
Stroke of Awareness
By Maya Bell | Photos by Donna Victor
Not long ago, physicians could not do much to alleviate the devastating damage wrought by an ischemic stroke. But today’s advanced clot-busting drugs and minimally invasive technology wielded by UHealth neurologists enable patients like Chase Anderson to resume an active, normal life.
 |
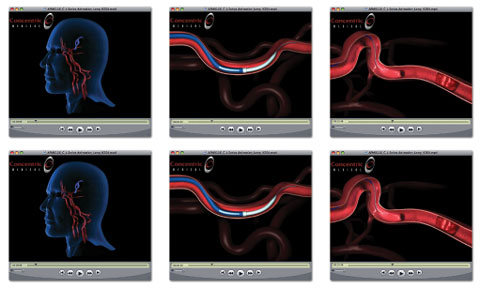
| Chase Anderson reviews MRI images with Dileep R. Yavagal, M.D., who treated his potentially debilitating
stroke. |
At 28, Chase Anderson prided himself on keeping fit. A lifelong athlete, he ate wisely, lifted weights, and jogged. Running up the tallest mountain near his Hong Kong home barely tired him.
The international businessman from Tennessee didn’t know what a stroke was, much less consider himself a candidate for one while vacationing in South Florida.
Yet, on the second day of 2009, just after Anderson set out on a late-morning jog, he went limp and crumpled to the ground. Unbeknownst to him, a large clot had settled inside an artery, blocking blood flow to the right side of his brain—starving it of blood and oxygen, imperiling his cognition and paralyzing his left side.
Instead of flying back to Knoxville that afternoon, Anderson and his family embarked on a harrowing medical odyssey where they discovered what many of the 750,000 Americans who annually suffer a blockage or rupture of a blood vessel in the brain learn: Stroke can afflict anyone, any time, often without warning, often without raising alarms that prompt urgent action.
Over the next few months, Anderson would also learn how blessed he was to have relented to his mother’s wish to vacation in South Florida. He believes he owes his amazing recovery
to Miller School neurologists at Jackson Memorial Hospital’s Comprehensive Stroke Center, where cutting-edge therapies and research are opening the next frontier in stroke treatment and prevention.
“I didn’t even want to be in Miami, but if I were somewhere else I would have been permanently damaged or even vegetative,’’ the devout Christian says from his mother’s Knoxville home. “God guided me here and saved my life.’’
About six hours after
Anderson’s collapse, Dileep R.
Yavagal, M.D., director of interventional neurology and a leader in the growing field of endovascular stroke therapy, inserted a thin wire with
a tiny corkscrew tip into Anderson’s thigh via a catheter. Threading the wire into Anderson’s brain, just past the clot, Yavagal released the tip, snagged the mass, and gently tugged it out, restoring blood flow to the right side.
Three days later, Anderson was reading cards and speaking Mandarin. Six months later, he could jog two miles.
“That’s a real success story,’’ says Ralph Sacco, M.D., M.S., professor and chair of neurology and a renowned expert on stroke prevention and treatment. “If that artery remained closed, I think he would have been seriously disabled or worse.’’
Just 15 years ago, there was little doctors could
do for acute stroke sufferers like Anderson, who was felled by an ischemic stroke. Accounting for almost
85 percent of stroke cases, they occur when a vessel
is clogged, blocking the flow of blood, vital nutrients, and oxygen to the brain. Hemorrhagic strokes, which account for about 15 percent of stroke cases, occur when a vessel ruptures and leaks blood into the brain.
But today, thanks to clot-busting drugs and tiny, minimally invasive devices that retrieve clots, plug aneurysms, and deliver medication directly
to the occlusion, neurointerventionalists like Yavagal have about eight hours to reverse ischemic strokes, saving brain cells, halting paralysis, and improving the prospect for recovery.
Miller School clinicians and researchers are confident that one day soon they’ll also be able to widen the treatment window beyond eight hours, pinpoint the genes that lead to stroke, and better prevent brain attacks and damage—perhaps by prescribing a pill made from an ingredient in wine.
“Before, if you had a stroke, we’d say, ‘Sorry, we’ll treat the symptoms and send you to rehabilitation, but there’s nothing else we can do,’” says Jose Romano, M.D., associate professor of clinical neurology and director of the stroke division.
“Now we can really make a difference and, for such a devastating disease, that is remarkable.’’
Making a difference depends on a system similar to a trauma network to ensure acute stroke patients reach Jackson quickly when they run out of treatment options elsewhere. In Anderson’s case, the system at the Comprehensive Stroke Center, the only one in Miami-Dade and Monroe counties, worked as designed.
His attack began shortly after he and his brother-in-law left their Key Biscayne hotel for a run. Suddenly, a sharp pain jolted the right side of Anderson’s head. His left arm began shaking. When he tried to talk, his words spilled out in a jumble.
Stumbling to a patch of grass, Anderson waited for the bewildering episode to pass. He had no idea he had just begun a race against the clock—that every minute two million brain cells were dying.
“I kept thinking, ‘I’m young and healthy and this can’t be that big of an issue,’’’ he recalls.
It was a very big issue. The third leading
cause of death, stroke is the No. 1 cause of chronic adult disability in North America. Seven of ten survivors cannot resume normal activities. Only about 5 percent reach a hospital fast enough to receive an intravenous dose of tissue plasminogen activator, tPA, the only clot-busting drug approved for the urgent treatment of ischemic stroke.
A major reason: Like Anderson, many people don’t realize they’re having
a stroke or recognize the symptoms. They include sudden weakness or numbness, especially in one arm, leg, or one side of the face; trouble speaking, understanding, seeing, or walking; and/or a severe headache. Often there is no pain.
To be effective, tPA must be administered
within four and one-half hours of the onset of a stroke, according to guidelines issued in May by the American Heart Association (AHA) and American Stroke Association. The updated protocol extends the three-hour treatment window adopted when tPA was approved in 1996. But Sacco, who will be the first neurologist to lead the AHA when he assumes the presidency in 2010, warns that the extra time does not relieve the urgent need to get to a hospital.
 |
In this promotional video from Concentric Medical, a manufacturer of clot retrieval devices, a thin wire inserted via a catheter from the thigh travels to a blood clot in the brain. Once the wire pushes past the clot, a corkscrew tip is deployed and the wire is gently tugged back out. As the wire retreats, the corkscrew tip snags the clot and pulls the mass out, restoring blood flow to the brain.
|
“We do not want to send the wrong message that now you have up to four and one-half hours,” Sacco says. “But rather, if someone comes in later than two or three hours, we still may be able to safely give the drug intravenously to improve outcomes.’’
The Drano-like drug also can be delivered by catheter directly to the clot within six or so hours, but few hospitals have skilled neurointerventionalists like Yavagal who can perform the delicate procedure. The Miller School has two, providing around-the-clock coverage at Jackson.
Mohammad Aziz-Sultan, M.D., assistant professor of clinical neurological surgery and neuroin-terventional radiology, shares the 24/7 responsibility with Yavagal, assistant professor of clinical neurology and neurological surgery. The founder of the Society of Vascular and Interventional Neurology, Yavagal says getting stroke patients to a comprehensive center within eight hours to benefit from the new catheter-device therapies remains a challenge, but the results make his unpredictable hours immensely rewarding.
“Once the window for IV tPA closes or IV tPA fails to open the blockage, neurointervention is the only treatment available to reverse stroke and paralysis,’’ he says. “That makes it worth it to be on-call half the year.’’
Fortunately, as Anderson sprawled on the grass, a passing physician recognized his symptoms and
he was rushed to Miami’s Mercy Hospital. There, he received tPA quickly.
Still, he did not improve. The clot failed to dissolve; his entire right lobe was at risk. Knowing they could do nothing more, doctors transferred Anderson to Jackson.
“He was part of the ‘drip and ship’ protocol,’’ Sacco says. “That’s when the drip—the IV tPA—occurs at another hospital, and if it’s not working, they ship the patient to a comprehensive stroke center, and that’s what we are.’’
One of 13 in Florida, Jackson earned its accreditation from the Florida Agency for Health Care Administration
in March. The designation made official what already was fact under Sacco’s leadership: The medical center’s stroke service is the largest and most comprehensive
in the region, ensuring the greatest chance of recovery.
It includes a rapid-response 24/7 acute stroke team, an eight-bed stroke unit with specially trained stroke-savvy nurses, a 24-bed neuroscience intensive care unit, and two endovascular suites.
“Few places have all of these,’’ Romano says.
Miller School researchers and clinicians also are involved in clinical trials and studies aimed at better predicting and preventing strokes. With improved drugs, doctors are equipped to treat modifiable risks. They include diabetes, high blood pressure or cholesterol, smoking, excessive alcohol consumption, obesity, and coronary artery disease.
Uncontrollable risks include age, gender, race, and
family history of stroke. Generally, men, African-Americans, and those over 65 are at increased risk.
In Anderson’s case, doctors assume a clot developed in his leg during his flight from China, where he manages his
family publishing distribution business, and shot to his brain through a hole in his heart while he jogged.
Anderson didn’t know he had the hole,
a patent foramen ovale, but PFOs alone are not
a risk factor. One of every four people is born with
the defect. However, immobility and dehydration associated with air travel is known to cause deep
vein thrombosis in the calf.
He has since had the hole repaired and is confident he is not a candidate for another ischemic stroke.
So, too, is Herbert Rosenstock, 68. A New
York jeans manufacturer,
he was unaware he had
a stroke until he called his office upon waking up at his Delray Beach vacation home in mid-February. His speech sounded normal to him, but his staff couldn’t understand a word. “Everyone knew something was wrong except me,’’ Rosenstock recalls.
Today, he credits his renewed comfort level to an exhaustive exam at
the UHealth neurology clinic five weeks later. One test detected plaque in his carotid artery and, at Sacco’s recommendation, he will undergo regular carotid scans. He has also changed his diet, lost 15 pounds, and doubled his cholesterol medication.
“It’s not just that they knew what they were doing,’’ Rosenstock said of his five-hour visit. “It’s that they were so thorough, so professional, and treated me with such respect. And what they found gave me peace of mind.’’
Despite the gains, doctors still cannot explain the cause of about a third of ischemic strokes. With the belief that genes play a role, UM researchers are collecting DNA samples from South Florida’s stroke patients for future analysis and, they hope, new therapies.
The samples will augment data from the pioneering Northern Manhattan Stroke Study Sacco established at Columbia University in 1990 to focus on stroke risks in minorities.
“Let’s say you have bad genes but do everything right. You may not be at high risk for stroke,’’ says Tatjana Rundek, M.D., Ph.D., associate professor of neurology and co-investigator on the Manhattan study who directs UM’s Clinical Translational Research Division.
“However, if you combine your genes with
bad habits, such as smoking or poor diet, you can promote those genes to express themselves. We’re trying to pinpoint the ‘risky’ genes so we can test new strategies to prevent the gene from causing stroke.’’
Others are searching for therapies to prevent strokes or limit damage after they occur, and Miguel Perez-Pinzon, Ph.D., professor of neurology/neuroscience, may have found one in red wine.
In 2006 the director of the Cerebral Vascular Disease Research Center and his associates discovered that pretreating the brains of rats with resveratrol,
a powerful antioxidant found in the skin of grapes, before a stroke greatly reduces injury after one.
The reason: Resveratrol activates an anti-aging enzyme that helps make brain cells more resistant to stress.
Now Perez-Pinzon believes that pills packed with resveratrol, or a more efficient chemical, will soon offer the same protection to humans at risk for stroke. They would include people with diabetes, high blood pressure, or other stroke risks, and those who suffer transient ischemic attacks, brief strokes that often are precursors to major strokes.
“The goal is to have people prone to stroke take it prophylactically—like every other day,” he says. “It’d be the same principle as taking an aspirin daily. It also could resemble the daily consumption of wine, only this would be more efficacious. You’d have to drink a lot of wine—several bottles a day—to get the same effect.’’
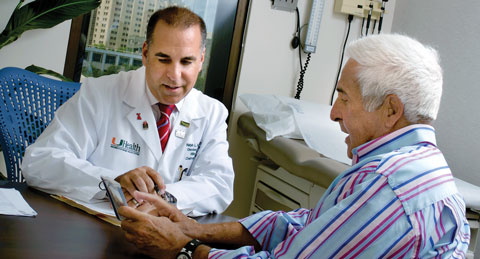 |
Ralph Sacco, M.D., M.S., chair of neurology, treats Herbert Rosenstock
to prevent him from having another stroke.
|
Perez-Pinzon owes his quest for natural stroke therapies to the primitive turtle. While pursuing
a master’s in marine biology at UM in the 1980s,
he was fascinated by diving turtles who could survive underwater with a single breath. How did their brains tolerate lack of oxygen, and could it
be replicated in humans?
The answer led Perez-Pinzon to the field of ischemic preconditioning, a phenomenon discovered in 1986 by cardiologists and neuroscientists who learned that a mild insult to the brain preconditions it against a subsequent, more lethal trauma.
“It’s kind of what happens when you exercise,’’ Perez-Pinzon explains. “If you exercise today you do better two days later, right? The same happens with the brain.’’
Now armed with a five-year $1.67 million
grant from the National Institutes of Health, Perez-Pinzon’s lab is trying to pinpoint the chemical mechanism by which resveratrol promotes ischemic preconditioning. If the scientists succeed, he believes they can replicate the process pharmacologically.
Until then, Perez-Pinzon and UM’s corps
of stroke specialists hope the public will remember the words of Chase Anderson.
“If stroke can happen to me, it can happen
to anyone, so it’s important to know the symptoms and risk factors,’’ Anderson says. “And if it happens, it’s important to know you can recover. You can get well and live a purposeful life again.’’
Using Albumin to Reduce Disability
from Stroke
Discovery often comes by accident, by stumbling on a surprise while pursuing another path. And it was by chance the eureka moment came to Myron Ginsberg, M.D., a UM neurologist and researcher seeking to prevent brain injury after stroke.
 |
| Myron Ginsberg, M.D., leads national trials testing the use of
albumin to prevent
brain injury
after stroke. |
Now the principal investigator
of a major National Institutes of Health clinical trial, Ginsberg was using albumin, a protein made by the body and concentrated in the blood, in the late 1990s as a control substance while testing another agent’s neuroprotective properties for a pharmaceutical company.
To his dismay, the substance in question had no effect on rats induced with stroke. But albumin, widely used to increase blood volume in shock patients, did. When injected intravenously, albumin reduced the size of the infarction, eliminated swelling and improved neurological function—even when treatment was delayed for four hours.
The pharmaceutical company insisted the results were a mistake. There must have been a mix-up. There wasn’t.
“We were the first to show
that albumin has special
beneficial properties that allowed
it to be protective,’’ said Ginsberg, the Peritz Scheinberg Professor
of Neurology. “Albumin doesn’t dissolve the clot, but it helps
to support blood flow in
the area of stroke.
It also combats dangerous free radicals that contribute to injury in stroke.”
To establish albumin as a safe therapy for humans, Ginsberg
and his team concluded a pilot trial with 82 patients in 2005.
The results were encouraging, thrusting the researcher more accustomed to the lab to the forefront of what he hopes will
be the first successful clinical
trial of a neuroprotective agent for humans.
Known as the Albumin in
Acute Stroke Trial, or ALIAS,
Phase III, the five-year, $25 million double-blind study is recruiting 1,100 patients in 60 to 70 North American hospitals. Despite some stops and starts, it holds the exciting promise of proving that albumin, either given alone or with the clot-busting drug tissue plasminogen activator (tPA), will reduce disability from stroke.
“If we show it works, people could start using it right away,’’ Ginsberg says. “It’s conceivable that one day you could even begin giving it in the ambulance.’’ |
|
 |
 |