Assessing FibroScan
Hepatitis B and C have the potential to produce liver scarring, so patients with the diseases need to get biopsies every few years to ensure that cirrhosis hasn’t developed.
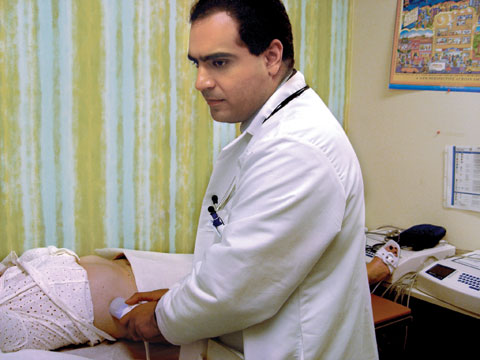 |
| Kaveh Sharzehi, M.D., a fellow with the Center for Liver Diseases, performs about 15 FibroScan assessments each week. |
The Miller School’s Center for Liver Diseases is now conducting a clinical trial on a new device that can detect liver scarring without biopsies, which are sometimes associated with significant complications and call for patients to remain under medical supervision a few hours afterward.
Known as FibroScan, the new technology utilizes ultrasound waves to disclose the scope and severity of hepatic fibrosis and is currently being evaluated for efficacy and patient safety by the U.S. Food and Drug Administration.
“We are using FibroScan within a research protocol,” says Eugene Schiff, M.D., director of the Center for Liver Diseases at the Miller School. “Until it’s licensed by the FDA, we can use FibroScan only on patients being seen at our medical center and by physicians working in the Division of Hepatology.
“From some standpoints, this will not give you the accuracy of a biopsy, which allows you to see inflammatory cells and what kind they are,” Schiff cautions. “And FibroScan would be worthless for detecting cancer.”
It’s an entirely different matter, though, when the objective is to detect the formation of fibrous tissue on the liver.
“FibroScan may be more accurate in terms of fibrosis, because it covers a much bigger area than a biopsy does,” Schiff says. He anticipates that FibroScan, which is made by the French company Echosens, will have little trouble gaining FDA approval.
|