|
In one of the first-of-its-kind studies conducted with humans, University of Miami researchers are attempting to reverse heart failure by injecting millions of lab-grown stem cells into scarred and damaged heart muscle.
If successful, the clinical trial led by Joshua Hare, M.D., director of the Interdisciplinary Stem Cell Institute at the Miller School of Medicine, could add years and improve the quality of life for the five million Americans who suffer from heart failure.
As patients and advocacy groups clamor for effective treatments for chronic diseases now considered incurable, Hare’s clinical trial is just one example of how the scientific community is using stem cells to help find ways to erase maladies afflicting millions. When given the right cue, stem cells harvested from adult bone marrow, umbilical cord blood, human placental tissue, and early-stage human embryos have the potential to become the type of tissue needed to repair a variety of organs.
Late last fall, researchers in Wisconsin and Japan reported they had been able to turn adult skin cells into cells as versatile as embryonic stem cells, but said more work is needed and embryonic stem cell research should not be abandoned.
 Of course, the Miller School isn’t turning a blind eye to the national debate about how, when, and in what quantity stem cells are used, but it’s carefully focusing new resources on cell-based therapies to find cures where none exist. Hare, a noted expert in the field, was recruited away from The Johns Hopkins University School of Medicine two years ago to help make the Miller School a leader in this fledgling field of regenerative medicine. Of course, the Miller School isn’t turning a blind eye to the national debate about how, when, and in what quantity stem cells are used, but it’s carefully focusing new resources on cell-based therapies to find cures where none exist. Hare, a noted expert in the field, was recruited away from The Johns Hopkins University School of Medicine two years ago to help make the Miller School a leader in this fledgling field of regenerative medicine.
The Interdisciplinary Stem Cell Institute is at the forefront of advancing the research by translating what has been learned in the laboratory into treatments for patients, Hare says, while most other centers still are focused on basic research.
“Working with stem cells has given us a lot of insights into biology in general and many potential new therapies for things that are not treatable now. I think this ranks among the most important new therapeutic developments of our time,” Hare says.
Across the Miller School campus, work is under way in various labs—including major work at the Diabetes Research Institute and The Miami Project to Cure Paralysis—exploring cell-based therapies for blood vessel diseases, diabetes, spinal cord and traumatic brain injury, bone and cartilage replacement, and diseases of the eye, among others.
The patients in the heart failure study will have some of their own bone marrow removed. In the lab, mesenchymal stem cells will be sorted out and multiplied under the direction of Ian K. McNiece, Ph.D., professor of medicine and director of experimental and clinical cell-based therapies within the institute. The cells are injected into damaged tissue during bypass surgery.
“For these people, there is nothing else available to them but a heart transplant,” Hare says. “But with this approach we might be able to restore them back to health.”
Mesenchymal stem cells secrete a number of growth factors and have been shown to seek out damaged or inflamed areas in the body and repair them. Hare was principal investigator for a previous multicenter study that infused donor mesenchymal cells into people who had recently had heart attacks and compared their outcomes with similar patients who did not get the stem cells.
“The stem cell patients had lower rates of side effects such as cardiac arrhythmias, and they had significant improvements in heart, lung, and global function,” Hare says. “Over five million people in this country are classified as having heart failure. That’s why we’re so excited about the study. It really addresses a major public health issue.”
McNiece, who worked with Hare at Johns Hopkins, says developing a cell-based therapy is more complex than developing a new drug and requires additional approval processes. “It’s very expensive and very complicated,” McNiece says.
“What attracted me to the University of Miami was the program that Josh was looking to build here,” he says. “One of the challenges is that it is very difficult to get money to do this type of work. UM is putting the resources in place to make these therapies a reality.”
To illustrate the complexity of the work, McNiece explains that when mesenchymal cells grow naturally inside the marrow of the bone, they adhere to a hard surface. Researchers had to figure out how to get them to grow without a surface to cling to and provide better engraftment in tissue such as the heart.
McNiece’s lab is currently housed in the Fox Building but will move into the new Biomedical Research Building when it is completed later this year. It will include ten rooms for manufacturing stem cells of various kinds for use by Miller School researchers and outside researchers as well.
Manufacturing Islet Cells to Cure Diabetes
Four teams of investigators from the Cell Transplant Center at the Diabetes Research Institute (DRI) have been wrestling with similar problems in the search for a cure for type 1 diabetes.
DRI researchers have been able to reverse diabetes by transplanting insulin-producing cells from cadavers into patients, but the supply is scarce and patients must take anti-rejection drugs. Over the years, the cells lose some function and replacements are needed.
Islet cells are tiny factories in the pancreas that churn out insulin to be released into the bloodstream as needed to convert sugars from food into energy for cells.
DRI researchers are attempting to get embryonic stem cells to become these insulin-producing cells by culturing them in the lab.
“We think embryonic stem cells are the most powerful, and in terms of their ability to expand over time, you can start with one million and can have two million the next day and then four million, and they have the ability to become any type of cell in the body,” says Juan Domínguez-Bendala, Ph.D., director of one of the stem cell laboratories at the DRI.
“But they don’t like to become insulin-positive cells, so you have to push them to do it. You have to mimic the environment in the pancreas where they would naturally develop,” he says.
The cells make up only 1 percent of the pancreas but use 25 percent of its oxygen supply, Domínguez-Bendala says.
“These cells need oxygen. We reasoned that if we are going to get islets from stem cells, we have to give it to them.”
Domínguez-Bendala and colleague Chris Fraker, M.S., senior research associate in the Tissue Engineering Laboratory at the DRI, created an innovative device to culture cells.
“We call this system an oxygen sandwich,” Domínguez-Bendala says. “When we used this with mouse embryonic stem cells, we saw almost a 100-fold increase in [insulin-producing] cells.” (To learn more about the device, read the story on page 16.)
Domínguez-Bendala and colleagues are also working on making human embyronic stem cells safe for transplantation. “If you have one cell that hasn’t differentiated into an insulin-producing cell, it can develop into a tumor, and you don’t want that. We’re working to engineer them to include a suicide gene, so that only these critical cells will survive,” he says. That work, supported by the Juvenile Diabetes Research Foundation (JDRF) and the Diabetes Research Institute Foundation, is still preliminary.
Because there are only a limited number of human embryonic stem cells available for federal research funding, the DRI created a separate lab to be able to work on embryonic cells that are not eligible for federal dollars.
“You have to keep the work totally separated,” Domínguez-Bendala says. “You can’t even use a microscope that was paid for by [National Institutes of Health] funding.”
In addition to funding from the JDRF, the DRI has received funding from UM’s Wallace H. Coulter Center for Translational Research and the American Diabetes Association to support research needed to create insulin-producing cells from embryonic cells. “Given the NIH restrictions, our work wouldn’t have been possible without them,” he says.
Camillo Ricordi, M.D., scientific director of the DRI, says he thinks much of the opposition to human embryonic stem cell research comes from a basic misconception.
Embryonic stem cells are created by combining egg and sperm in a lab dish—in vitro fertilization—to aid infertile couples who want to have a child. Extra fertilized eggs are frozen for later possible use by the couple, but often are not needed, and 400,000 are in storage in this country. Thousands of those will be thrown away.
“A lot of people don’t understand. They see little babies in test tubes, but this has nothing to do with suppressing life,” Ricordi says. “Our perspective is there is a moral obligation. If you have tissue that could save millions, then you should use it. The moment you throw away in a trash can material that will never become a human being, that would be criminal,” Ricordi says.
Repairing Spinal Cord Injuries
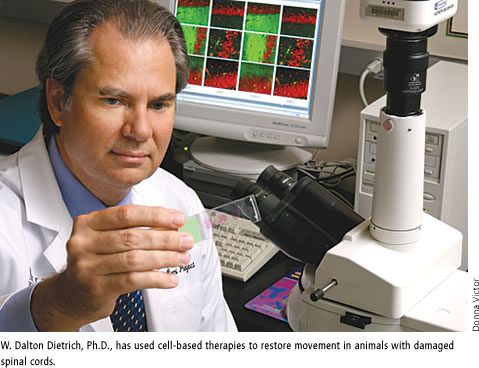
W. Dalton Dietrich, Ph.D., scientific director of The Miami Project to Cure Paralysis, who has been successful in using cell-based therapies to restore movement in animals with damaged spinal cords, hopes to get clearance from the U.S. Food and Drug Administration to begin clinical trials in humans later this year.
“Hopefully we will be able to start a Phase I safety trial with about 15 spinal-cord-injured subjects,” Dietrich says.
The clinical trial will be based on successful work in rats using a triple-combination therapy including Schwann cells—a type of glial cell—transplanted into a contused spinal cord, the most common cause of paralysis in humans. Twelve weeks after the combination treatment began, the rats were able to walk at about 70 percent of their normal ability.
Schwann cells can be taken from the patient’s own body and grown in the laboratory so there is less danger of rejection. However, in spinal cord injury the body produces substances that prevent repair of the damage, so just providing new supportive cells may not be enough.
In rat studies, Mary Bunge, Ph.D., and Damien Pearse, Ph.D., had to discover new ways of increasing a naturally occurring molecule called cyclic AMP, which helps enable the growth of nerve fibers. They treated the rats with the drug rolipram before and after transplanting the Schwann cells. The drug helps to promote growth of nerve fibers past the injury site and into the spinal cord on the other side.
Dietrich says the FDA wants separate safety studies on Schwann cells and rolipram before allowing a trial of the triple-combination therapy.
Researchers at The Miami Project also are trying to come up with cellular-based treatments for the one million patients each year who suffer traumatic brain injury, including soldiers injured in Iraq. The U.S. Department of Defense is funding some of the work.
Battling Eye Disease
Researchers at Bascom Palmer Eye Institute are using cell-based therapy in an attempt to halt deterioration of the eye caused by an inherited disease, retinitis pigmentosa, which damages retinal cells, says M. Elizabeth Fini, Ph.D., research professor at the McKnight Vision Research Center and Walter G. Ross Chair in Ophthalmic Research.
Participants in a Phase II trial led by Byron Lam, M.D., professor of ophthalmology, receive an implant of a tiny device containing human retinal epithelial cells genetically modified to secrete a growth factor, ciliary neurotrophic factor, over sustained periods of time.
“CNTF growth factor has been shown to protect the retina,” Fini says. “The Phase I trial was just a safety trial, but some patients said they could see better, and that was amazing.”
A similar trial led by Thomas Albini, M.D., is under way for patients who suffer from the dry form of age-related macular degeneration, the leading cause of blindness in people over 55.
“It’s very exciting because there is no treatment for dry AMD, and it affects huge numbers of people,” Fini says.
Bascom Palmer doctors have also been able to heal corneas damaged by chemical burns and other assaults by applying amniotic matrix tissue from donor placentas to the damaged area. The tissue contains stem cells that promote healing while suppressing inflammation and scarring.
Restoring Vascular Health
Stem cells play a role in protecting the heart and blood vessels, but in diseases such as diabetes and with natural aging they can lose their ability to perform as intended. Keith Webster, Ph.D., director of the Vascular Biology Institute, and colleagues are re-engineering defective stem cells to restore them to normal function.
“The institute has developed unique techniques for engineering stem cells with therapeutic genes that can be used to replace diseased or damaged vessels in patients with diabetes-related conditions, including ischemia of the limbs, heart, lung, and kidney,” Webster says. Researchers obtain blood samples from patients with vascular disease undergoing angioplasty and check for stem cell defects.
“Our vessels are in a constant cycle of damage and repair, and if the stem cells that are doing the repair are defective, the condition will continue to deteriorate,” Webster says.
“We have patented technology to replace defective stem cell genes with functional ones that will return normal function to the cells and make them better therapeutic agents,” he says. “Our goal is to isolate cells from patients, culture them in the lab, replace the defective genes safely, and return the genetically engineered cells back to the patient.”
Generating Replacement Tissue
H. Thomas Temple, M.D., vice chair and professor of orthopaedics and pathology and director of the University of Miami Tissue Bank, expects to see a day when replacement bone, cartilage, and tendon can be grown from stem cells.
“In the work I do, we remove large segments of bone and cartilage, and ideally we’d like to replace them with the real thing rather than a metal implant,” Temple says. “The clinical application of that isn’t here yet, but we’re working on it.”
For several years, surgeons have been able to use bone, cartilage, and tendon from cadaver donors to replace and repair areas damaged by injury or disease, but Temple says the repair takes a long time to heal and in some cases never does.
“If we were able to implant that bone with the patient’s own stem cells, we think the healing would be shorter and more complete. I think it’s very exciting and that it offers a lot of hope for patients.” |