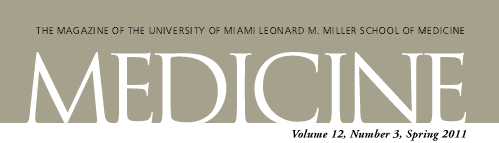|
 |
Chronic Questions
By Maya Bell
Miller School researchers are examining the many mysteries of wounds that refuse to heal, bringing relief to patients who have often suffered for years and uncovering cellular secrets that could ultimately lead to regeneration not just of skin, but entire organs.
 |
| Top left and top right - Zhao-Jun Liu, M.D., Ph.D.; Bottom left - Paolo Romanelli, M.D.; Bottom right - courtesy of Evangelos Badiavas, M.D., Ph.D. |
The largest organ in the human body, skin has amazing healing powers. Even when it has been slashed to the bone, a carefully choreographed sequence of complex and overlapping biochemical events immediately begins to repair the damage.
Within minutes, platelets form a clot to control bleeding. Within hours, white blood cells engulf bacteria and dead tissue, reducing the risk of infection. Within days, endothelial cells dispatched from bone marrow help rebuild severed blood vessels while fibroblasts divide and migrate, laying down a collagen matrix on which new cells multiply, filling in the wound bed. Within weeks, the wound is covered and the tissue begins to mature, regaining up to 80 percent of its original strength over the next year.
As in a perfectly synchronized orchestra, each player cooperates with the others to repair the dermis, the lower layer of skin, and the epidermis, the outer layer. In fact, the cells know their roles so well that, in the lab, researchers can break skin down to its individual components, reassemble them, inflict a wound, and the skin still heals. Researchers have even returned skin cells to their embryonic state, where they have the potential to form a new heart, lung, or other organ.
Yet, despite skin’s seemingly magical capacity for rejuvenation and reinvention, nearly 6 million Americans suffer from chronic wounds—usually venous, pressure, diabetic foot, or arterial ulcers—that fail to heal sometimes for months, often for years.
Painful, disabling, and leading to millions of amputations, chronic wounds cost the U.S. health care system an estimated $20 billion a year and exact a terrible emotional and physical toll on people like Karen Marshall, a registered nurse who lives in Orlando. Her wounds, which circle her ankles and expose her tendons, forced her to retire five years ago, at age 57, and have consumed her life, and health, ever since.
 |
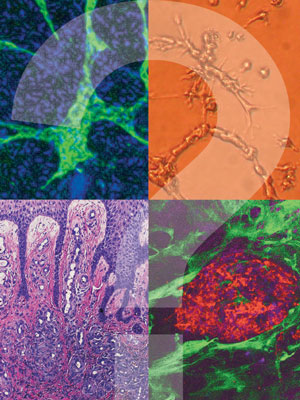
| From left, Evangelos Badiavas, M.D., Ph.D., research associate Marcela Salgado, M.D., research fellow Alejandra Vivas, M.D., and Jose Ollague, M.D., examine a chronic leg wound of a patient who began to heal after receiving an injection of his own stem cells through the NIH trial Badiavas is leading. |
“My legs hurt so badly I couldn’t touch them with a feather,” Marshall says.
But fortunately for Marshall, whose wounds are a symptom of a systemic inflammatory disease known as CREST syndrome, her wound-care specialist referred her to the University of Miami Health System and the Department of Dermatology and Cutaneous Surgery, where she is participating in a promising clinical trial sponsored by the National Institutes of Health and the Miller School’s Interdisciplinary Stem Cell Institute. The study is designed to show that stem cells extracted from a patient’s own bone marrow and injected into his or her non-healing wounds can help close the most stubborn sores.
Led by Evangelos Badiavas, M.D., Ph.D., associate professor of dermatology and a member of the stem cell institute, the study is open to people whose wounds have failed to heal for at least a year with standard treatments, including skins grafts, compression dressings, debridement (the removal of dead skin and tissue), or hyperbaric oxygen therapy, which saturates the blood with oxygen.
For Marshall, who has tried all the treatments, the drive to Miami is arduous, the brief hospital stay stressful, and the recovery from what is usually an outpatient procedure slow. But she is eagerly participating because, after her second stem cell injection, she began to see the first signs of healing.
 |
| Omaida Velazquez, M.D., and
Zhao-Jun Liu, M.D., Ph.D., are conducting translational research designed to identify novel ways to more effectively mobilize endothelial progenitor cells, which spur blood vessel formation and tissue repair, to heal ulcers in patients with diabetes. |
“It’s tremendous,’’ Marshall says. “I’ve been living with this for almost six years. But now my wounds are covered with granulation tissue. They’re not pretty, but it sure looks good.’’
The nation’s premier wound healing and infection research center, the department has been at the forefront of groundbreaking clinical care and translational research that have bettered the lives of millions since its founding more than 53 years ago. It is renowned for developing the first porcine model crucial to simulating human wound healing, pioneering the use of occlusive dressings and tissue-engineered skin, and detecting the presence of antibiotic-resistant biofilms in wounds.
Now home to a new, state-of-the-art wound care center at University of Miami Hospital and an array of clinical trials led by Vice Chairman Robert Kirsner, M.D., Ph.D., Stiefel Laboratories Chair and Professor of Dermatology, the department also boasts a dermatopathology service that evaluates more than 35,000 wound biopsies a year and research laboratories that test the most promising dermatologic pharmaceuticals.
It also has produced many of the nation’s wound healing experts. Some, such as Kirsner, remained at the Miller School after graduation; others, like Badiavas, recently returned. Now they’re joining forces with investigators at the Diabetes Research Institute and The Miami Project to Cure Paralysis, as well as vascular surgeons, biomedical engineers, and microbiologists across campus to pursue multiple goals. Among them: identify the molecular defects that inhibit healing, curtail the escalating rate of amputations, develop better wound-healing and infection-control strategies, and—one day—recruit the regenerative powers of skin to the battles against paralysis, cancer, and other diseases.
 |
| Marjana Tomic-Canic, Ph.D., is seeking tissue repair biomarkers that could spur development of tests to quickly diagnose non-healing wounds; research professor Stephen C. Davis is currently working on ways to break up or prevent biofilms, walls of bacteria that cause infection and prevent healing. |
“This institution is very special because we go from soup to nuts—from basic sciences to laboratory and animal research to clinical,’’ says Kirsner, founding board member and former president of the Association for the Advancement of Wound Care and co-editor of Wound Healing, the definitive text on assessment and treatment strategies. “Most institutions can’t do that. They may have a great basic science researcher somewhere, but they don’t have it all in one setting. It makes UM very special.’’
UM’s special role will assume even greater importance as the risk factors for chronic wounds—particularly aging, obesity, and diabetes—continue to rise. Targeting people with poor circulation and thus insufficient oxygen in their blood, chronic wounds can develop after surgery or radiation. They also can erupt from various syndromes like the one that afflicts Marshall, or genetic disorders like epidermolysis bullosa (EB), a painful and disfiguring disease caused by mutations in the proteins that hold skin together.
But the vast majority of chronic wounds are classified as venous, pressure, or diabetic ulcers. Usually found on the legs, venous ulcers are thought to develop from damaged valves in the veins. Often called bedsores, pressure ulcers are common in paralyzed or otherwise immobile patients whose skin is squeezed between bone and another surface, such as a bed or wheelchair.
Usually on the bottom of the foot, diabetic foot ulcers are the leading cause of amputation. Every 30 seconds, a doctor somewhere in the world is amputating a diabetic’s infected toe, foot, or leg. With the incidence of type 2 diabetes expected to double over the next decade, that astonishing statistic also is expected to explode, in part because many diabetics live with high levels of blood sugar for years before they are diagnosed.
By then, damage to blood vessels and nerves can numb their feet to the point they wouldn’t notice stepping on a nail. John H. Bowker, M.D., professor emeritus of orthopaedics who established South Florida’s first diabetic foot clinic at Jackson Memorial Hospital in 1982, recalls one patient who suspected a needle was imbedded in her foot. Bowker, editor of Levin and O’Neal’s The Diabetic Foot, the gold standard in diabetic foot care, indeed found the needle—and two others.
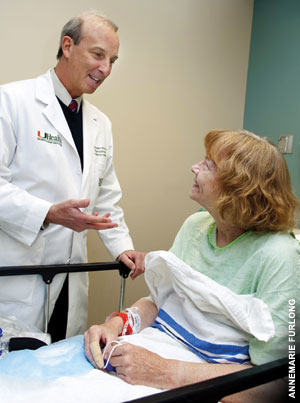 |
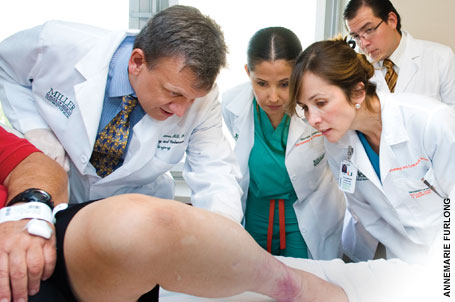
| Robert Kirsner, M.D., Ph.D., vice chair of the Miller School’s Department of Dermatology and Cutaneous Surgery, chats with patient Karen Marshall, who makes regular visits from Orlando to participate in the department’s stem cell injection trial. |
Such undetected injuries often become infected, sometimes requiring amputations within the foot to salvage surrounding healthy tissue and preserve the ability to walk.
But across the University, researchers like Marjana
Tomic-Canic, Ph.D., professor of dermatology and director of the Wound Healing and Regenerative Medicine Research Program, are teaming up with clinicians like Kirsner and
Badiavas to determine how the tissue repair process goes awry in chronic wounds, and with basic scientists like Tongyu Cao, Ph.D., research assistant professor, and Jie Li, M.D., Ph.D., research associate professor, to figure out how to reverse it.
The answer, Tomic-Canic says, could have profound implications for patients like Marshall, as well as those who are paralyzed or stricken with cancer. After all, she notes, cancer is akin to wound-healing run amok. To feed tumors, cancer cells proliferate, migrate, and sprout new blood vessels—just like normal healing wounds.
For now, Tomic-Canic is developing a new protocol to halt the alarming rate of amputations. She and her lab team found that cells on the edges of chronic diabetic foot ulcers include one or two proteins that aren’t present in ulcers that heal with standard therapies.
Once she confirms a correlation between the biomarkers and non-healing outcomes, clinicians could use an on-the-spot test to distinguish ulcers that still have the potential to heal from those that don’t. Such a test could revolutionize wound care, expediting more aggressive biological treatments for non-healing wounds and saving countless limbs.
Omaida Velazquez, M.D., professor of surgery and chief of the Division of Vascular and Endovascular Surgery, and her research associate, Lee Goldstein, M.D., assistant professor of surgery, also have made key discoveries that one day may harness the power of endothelial progenitor cells (EPCs) to heal diabetic ulcers. Stored in the bone marrow, EPCs are summoned from the marrow by hormones in the wound to help form new blood vessels and release pro-healing molecules at the wound site. But Velazquez and Goldstein found that the marrow in diabetics fails to dispatch EPCs to the wound, halting angiogenesis—the growth of new vessels from existing ones—and, consequently, wound healing.
Discerning a partial remedy, they learned that hyperbaric oxygen kicks EPCs out of the marrow and into the bloodstream. “In fact, with hyperbaric oxygen, the messenger that causes EPCs to be released skyrockets in the marrow,’’ Goldstein notes. “And as soon as you activate the messenger, the number of EPCs in the blood triples, and the wounds heal.’’
At least they do in healthy individuals. Diabetics, however, appear to have another critical deficiency, this one in the amount of signaling proteins that summon circulating EPCs to the wound so they can begin rebuilding severed vessels. But Velazquez’s lab has isolated one signaling protein crucial to diabetes. She is optimistic it can be replicated pharmacologically and applied directly to the wound, triggering the healing process by attracting EPCs.
“If you bring more EPCs to a wound, you’ll get better blood vessel supply, which brings nutrients and carries out waste and the wound heals more quickly,’’ Velazquez says. “That’s the edge right now between our translational
research and patient care.’’
Research professor Stephen C. Davis has been narrowing that edge since joining the Miller School in 1986.
A member of the team that helped chairman emeritus
William H. Eaglstein, M.D., and professor emeritus Patricia Mertz develop the first and best-established porcine model to simulate human wound healing, Davis has conducted efficacy tests for dozens of pharmaceutical wound healing products found on drugstore shelves today.
He also helped the U.S. military develop the first topical oxygen emulsion. Applied to acute surgical or burn wounds, the emulsion significantly stimulated healing in pigs and was later sent to soldiers in Iraq. Since the suspension stimulated collagen production and blood vessel formation, it may have implications for oxygen-deficient chronic wounds as well.
Equally important to people like Karen Marshall is Davis’s work to find a way to break up or prevent biofilms, which form when bacteria link together, building impenetrable walls against antimicrobial agents. As Marshall has learned from the multiple staph infections she has acquired over the last five years, chronic wounds like hers are ideal homes for biofilms.
Twice she’s had to postpone stem cell injections to battle infection, but she remains as upbeat as Badiavas is confident that his cell-based therapy will help patients like her put their misery behind them. His confidence is born from the remarkable success he has achieved since he injected his first patient with bone marrow-derived stem cells a decade ago.
Just two weeks later, the gaping belly wound the man had endured for seven years after emergency gall-bladder surgery sported a distinct blush of redness, an indication of angiogenesis. Soon the wound closed completely. “It was remarkable,’’ Badiavas recalls. “After seven years, he healed.’’
Though the stem cell trial that evolved from that initial test is in its infancy, Badiavas, Kirsner, Tomic-Canic, and Davis are already planning the next trial, this one to test the use of donor bone marrow stem cells on the chronic wounds of patients whose own stem cells are likely compromised, such as children with EB, the dreadful disease that can produce open wounds from the gentlest touch.
A pediatric dermatologist, department Chairman Lawrence Schachner, M.D., the Harvey Blank Professor of Dermatology, still recalls a lecture by Kirsner and Davis that sparked the department’s pioneering application of Apligraf, bioengineered skin derived from the discarded foreskins of newborn boys, on a baby girl born with the telltale
red blisters covering her body. She improved dramatically, and EB sufferers had a life-changing treatment.
Given the dedication and expertise of UM’s wound healing team, Schachner is certain they and their collaborators in other departments will produce more stunning achievements. “It’s very possible that, in the future, you’ll give us some skin cells with a couple of hair follicles and a bit of fat, and we’ll give you a new hip,’’ Schachner says. “This is not Star Wars; that is the potential. Diagnostically and therapeutically, we will continue to do incredible good for a huge number of people.’’
Maya Bell is senior editor at the Miller School of Medicine. |
 |
 |